
Slaked Lime Formula (Ca(OH)₂): Properties, Production, and Applications

Slaked lime, also known as calcium hydroxide, is a widely used inorganic compound with significant applications in construction, agriculture, and environmental protection. This article provides an in-depth yet concise overview of its chemical formula, production process, properties, and common uses.
Slaked Lime Formula Chemical
The chemical formula for slaked lime is:
Ca(OH)₂
Ca stands for calcium, a metal in Group 2 of the periodic table.
OH⁻ is the hydroxide ion, a basic anion.
One calcium ion (Ca²⁺) bonds with two hydroxide ions (OH⁻) to form calcium hydroxide.
How Slaked Lime Is Produced
Slaked lime is formed by reacting quicklime (CaO) with water. The reaction is exothermic, meaning it releases heat:
CaO + H₂O → Ca(OH)₂ + heat
This simple yet vigorous chemical reaction transforms quicklime (also called burnt lime) into slaked lime.
Physical and Chemical Properties of Calcium Hydroxide
| Property | Description |
|---|---|
| Appearance | White powder or crystalline solid |
| Solubility | Slightly soluble in water (forms limewater) |
| pH Level | Strongly alkaline (basic) |
| Reaction with CO₂ | Forms calcium carbonate (CaCO₃) |
| Thermal Behavior | Decomposes at high temperatures to form CaO |
Cronus has innovated the design and optimized the configuration of a new generation of calcium oxide (quicklime) digestion equipment based on more than ten years of practical experience in lime digestion and pulse bag dust collectors. It is one of the first and necessary special supporting environmental protection equipment for large-scale calcium hydroxide powder processing enterprises (groups).
Capacity: 15~50t/h
Application scenarios:
The construction industry produces slaked lime for construction, masonry, plastering, etc. The chemical industry is used to produce calcium hydroxide and other chemical products. The environmental protection industry is used for environmental protection projects such as wastewater treatment and flue gas desulfurization. The metallurgical industry is used as auxiliary materials in the smelting process.
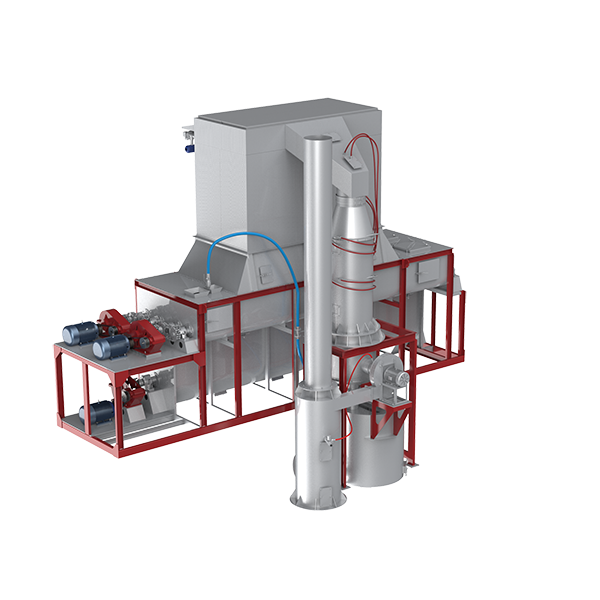
Calcium hydroxide equipment: working principle of lime digester
After the sieved calcium oxide (quicklime block) is crushed, it is lifted to the raw material warehouse (also known as lime storage warehouse) for standby;
When the digestion is started, the calcium oxide (20~40mm block material) is quantitatively and uniformly fed into the lime digestion box through a vibrating feeder, and the feeding speed can be adjusted at will;
Natural water (H₂O) is added, and the calcium oxide block material absorbs water for 5~8 seconds to produce a chemical reaction, gradually converting into calcium hydroxide (Ca(OH)₂), and at the same time a large amount of hot steam is produced, and a certain amount of calcium hydroxide powder is entrained.
Under the action of the fan, the hot steam is discharged outward through the pipeline, and the installed spray nozzles and spray nozzles form a water curtain wall section of a certain width and height under the action of the dust removal water pump. After filtering the dust in the hot steam, it is discharged into the atmosphere through the pipeline.
Calcium hydroxide equipment: product features of lime digester
The system is connected in series with pulse dust removal and water mist dust removal, with double protection. Bag damage can also avoid immediate shutdown and staggered maintenance and repair.
Each working axis is equipped with an independent dedicated reducer and motor for stable operation. Accurate weighing feeding and metering water distribution.
The patented water mist dust removal effectively recovers heat, forms water for promoting digestion, and takes into account the dust removal function.
The inner warehouse wall is equipped with a special cleaning nozzle to effectively avoid the problem of dust adhesion and calcification.
The use of combined digestion module technology greatly reduces the primary steel structure/foundation/factory building investment cost of the production line (less nearly 1 million), easy installation and maintenance, production capacity and equipment volume can be customized, with its own insulation layer and temperature sensing device, structural breakthrough design, few uncontrollable heat dissipation points, excellent temperature maintenance ability
Two-stage digestion design, low energy consumption, only three sets of variable speed drive devices, this level of volume is equivalent to the market 7-level digestion volume.
Comparison: Quicklime vs Slaked Lime
| Feature | Quicklime (CaO) | Slaked Lime (Ca(OH)₂) |
|---|---|---|
| Also Known As | Burnt lime / Calcium oxide | Hydrated lime / Calcium hydroxide |
| Reactivity with Water | Reacts violently, produces heat | Already reacted with water |
| Texture | Hard, dry solid | Soft, fine powder |
| Safety | Strongly caustic | Mildly caustic |
Conclusion
Slaked lime, with its simple formula Ca(OH)₂, plays a vital role in both industrial and environmental processes. Understanding its chemistry and real-world utility helps us appreciate how basic compounds can solve complex challenges in construction, pollution control, and agriculture.


