
Formation of Calcium Hydroxide: Master the Revolutionary Process for Diverse Applications
Calcium hydroxide, a white, odorless powder, has the chemical formula Ca(OH)₂. It is an important alkaline compound that is used in a wide range of applications including industry, agriculture, and the environment. Understanding how calcium hydroxide is formed is important to understanding its properties, uses, and importance.

Laboratory synthesis
In a laboratory setting, calcium hydroxide can be synthesized by a simple chemical reaction, i.e., the Formation of calcium hydroxide.
CaO + H₂O → Ca (OH)₂.
Calcium oxide (CaO), also known as quicklime, reacts with water (H₂O). The reaction is highly exothermic, releasing a large amount of heat.
When quicklime is added to water, a violent reaction occurs, causing the water to boil and release steam. As the reaction proceeds, the quicklime gradually dissolves, forming calcium hydroxide. The resulting solution, commonly called limewater, is a saturated solution of calcium hydroxide dissolved in water. Limewater is clear and colorless, but reacts with carbon dioxide in the air to form calcium carbonate, turning it milky white. These properties are used in various chemical experiments to detect the presence of carbon dioxide.
Industrial production
The production of calcium hydroxide is a more complex process on an industrial scale.
It begins with the extraction and processing of limestone, which is composed of calcium carbonate (CaCO₃), which is mined from a mine and then crushed into small pieces.


These pieces are placed in a lime kiln and heated to high temperatures, usually around 900-1000°C. At high temperatures, the calcium carbonate decomposes, releasing carbon dioxide (CO₂) and leaving behind calcium oxide.
CaCO₃→CaO+CO₂↑
Once calcium oxide is formed, it is further processed to form calcium hydroxide. This is achieved by adding water to the calcium oxide, a process called digestion. The digestion process is carefully controlled to ensure that enough calcium hydroxide is produced and to control the heat generated during the reaction. The calcium hydroxide produced can be dried and ground into a fine powder for use in various industrial fields
Introduction to CRW calcium hydroxide production line
Calcium hydroxide production line: covers raw material storage and pretreatment, quicklime digestion reaction, product separation and drying, tail gas treatment and emission control. The working principle of the equipment involves raw material preparation, limestone calcination, digestion reaction, product separation storage and tail gas treatment.
Its production is divided into wet method and dry method according to the process. The equipment includes crusher, vibrating screen, environmentally friendly lime kiln, screw conveyor, etc.
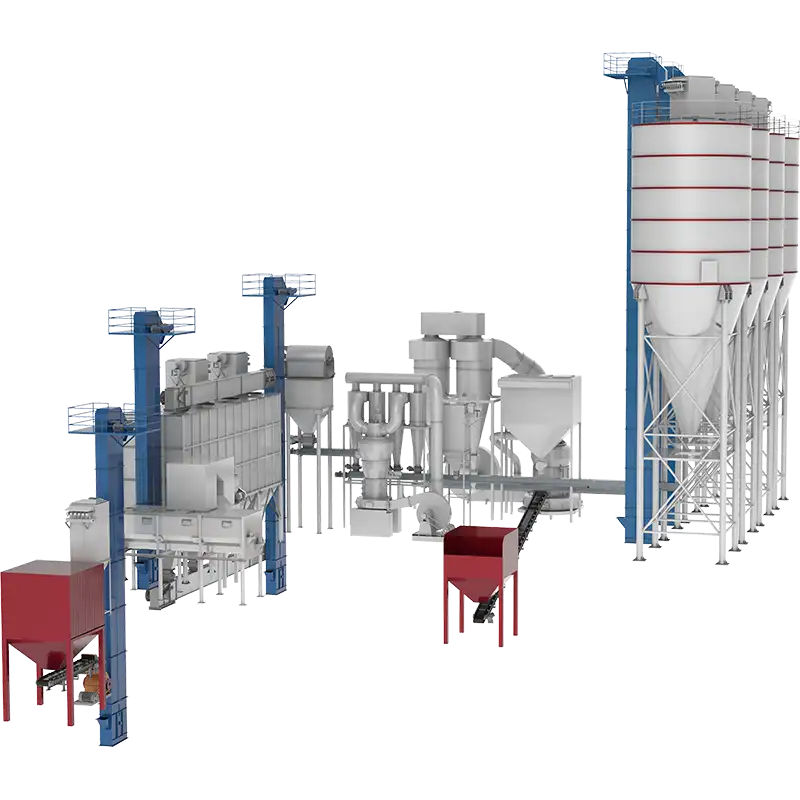
Production capacity: 8~50t/h
Finished product fineness: 200~800 mesh
Specific surface area: (BET) 17~23m²/g (without additives), ≥40m²/g (with additives).
The CRW series calcium hydroxide production line is a finished powder lime deep processing technology developed and designed based on the characteristics and uses of calcium hydroxide powder and gray calcium powder. It can produce high-purity, high-fineness, high-activity, high-specific surface area and high-porosity calcium hydroxide. It can meet the personalized needs of different customers for calcium hydroxide production line equipment.
CRW calcium hydroxide production line customer site





Choosing a CR calcium hydroxide production line requires comprehensive consideration of factors such as technical indicators, supplier qualifications and after-sales service. Kronos Machinery Company has significant advantages in equipment performance, environmental protection and energy saving, intelligence and after-sales service, and can provide customers with stable and efficient production solutions. For enterprises, choosing a supplier with technical strength and high-quality services can greatly improve production efficiency and stabilize product quality


